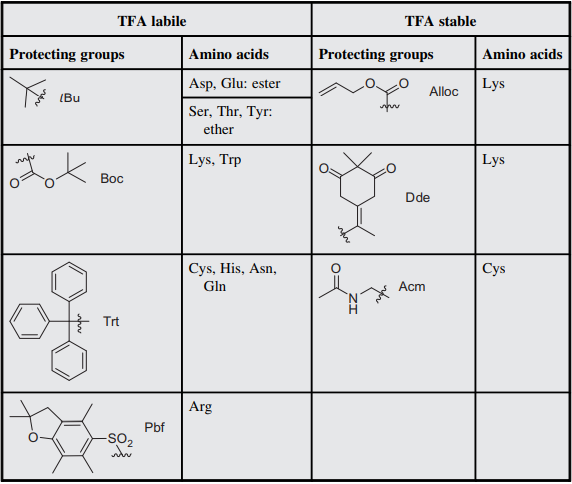
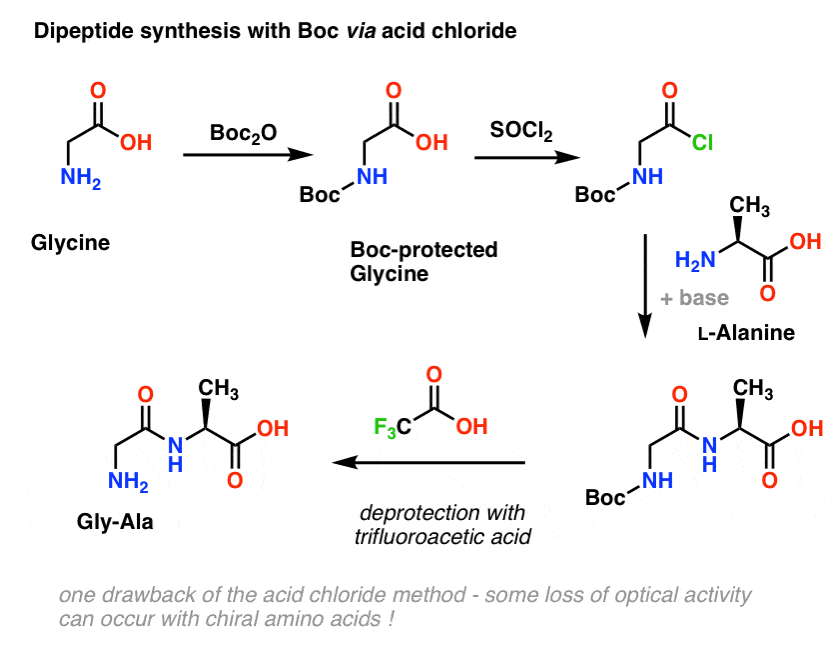
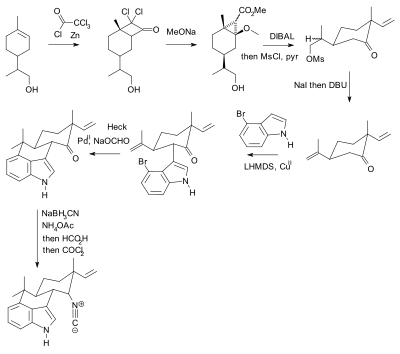
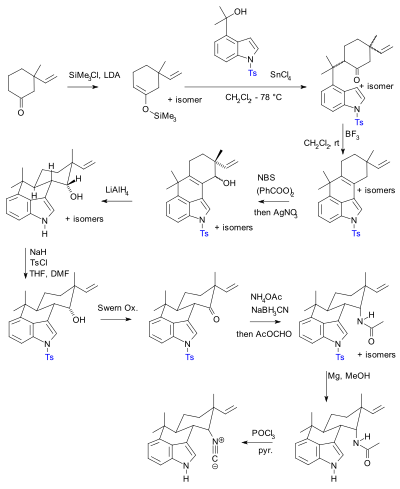
Molecules | Free Full-Text | Efficient Fmoc-Protected Amino Ester Hydrolysis Using Green Calcium(II) Iodide as a Protective Agent
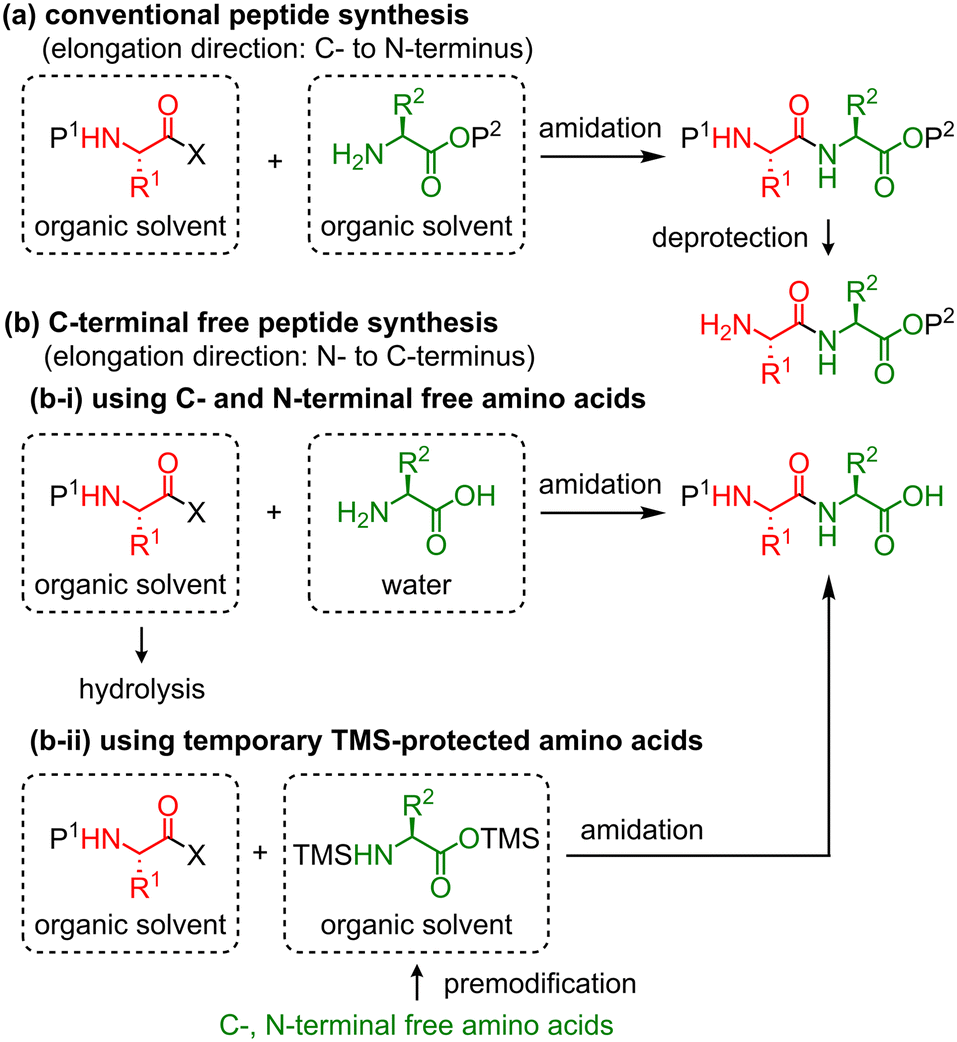
A liquid-phase continuous-flow peptide synthesizer for preparing C-terminal free peptides - Reaction Chemistry & Engineering (RSC Publishing) DOI:10.1039/D2RE00453D
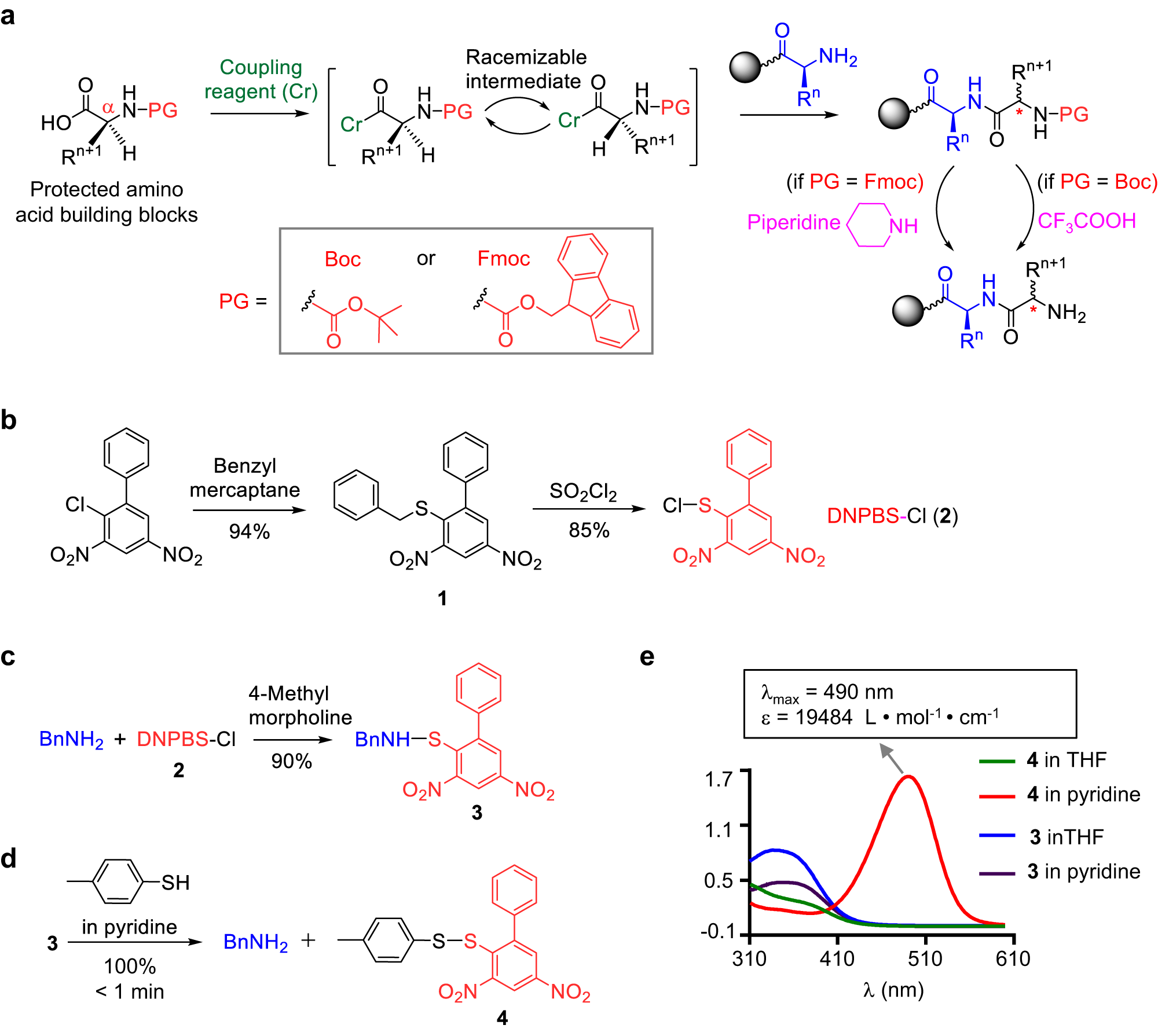
Suppression of alpha-carbon racemization in peptide synthesis based on a thiol-labile amino protecting group | Nature Communications

Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups | Nature Communications