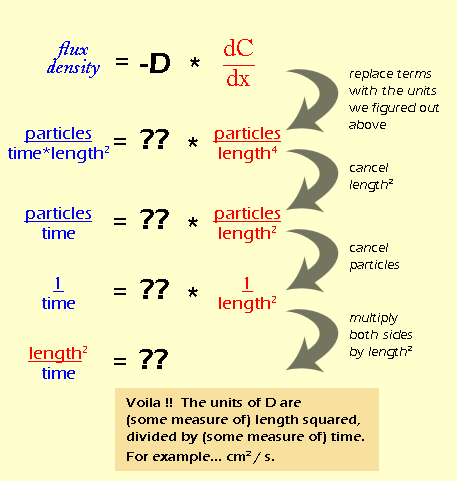
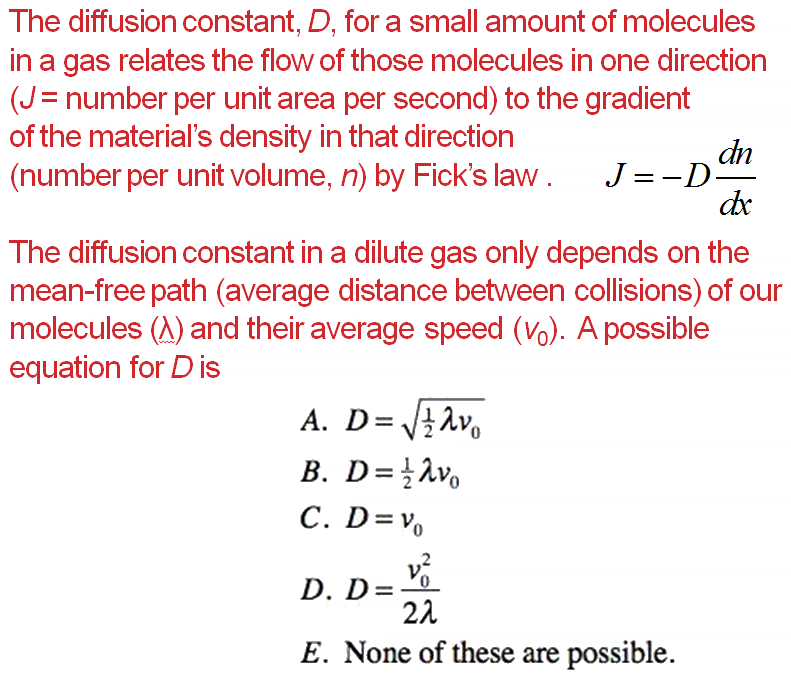
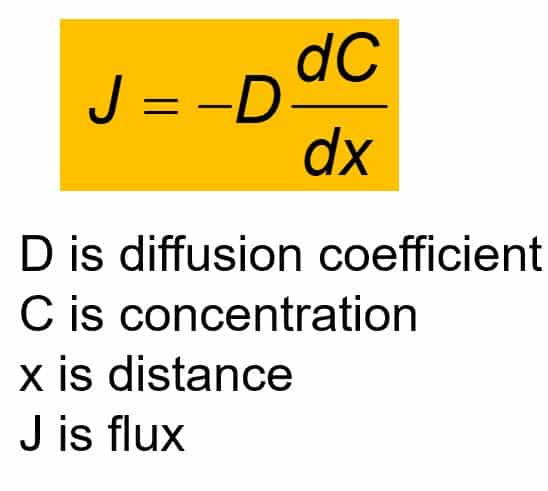
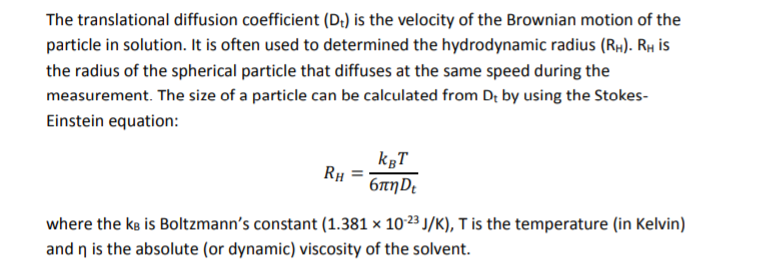
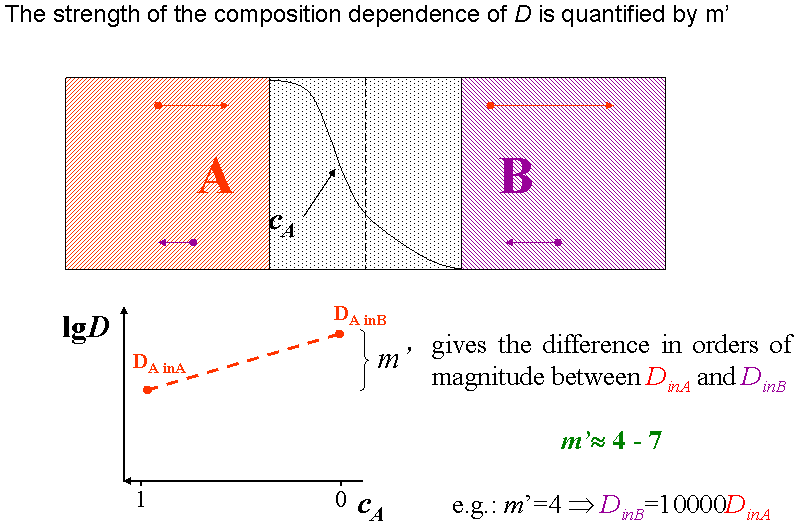
The diffusion coefficient of an ideal gas is proportional to its mean free path and mean speed. The absolute temperature of an ideal gas is increased 4 times and its pressure is

The diffusion coefficient of an ideal gas is proportional to its mean path and mean speed. The absolute temperature of an ideal gas is increased 4 times and its pressure is increased