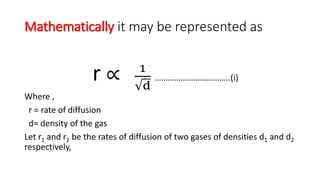
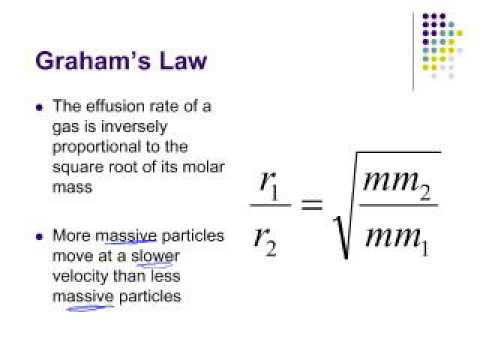
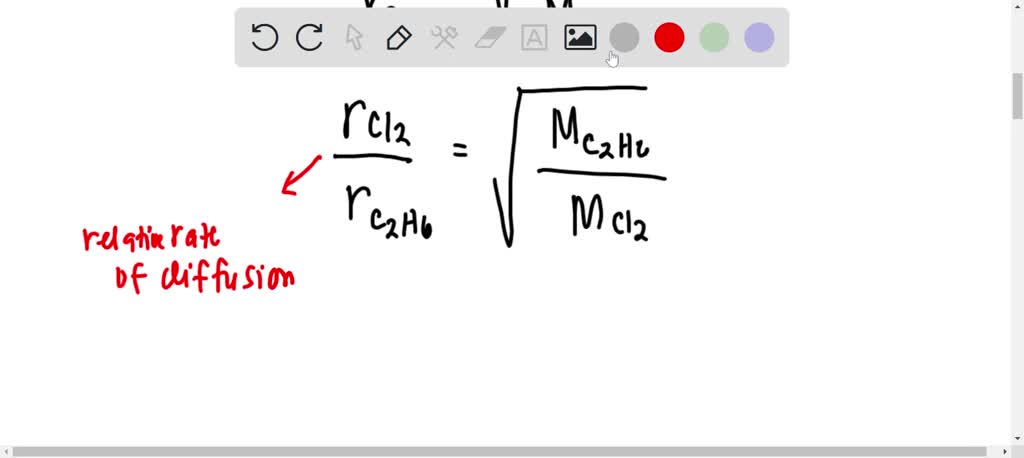
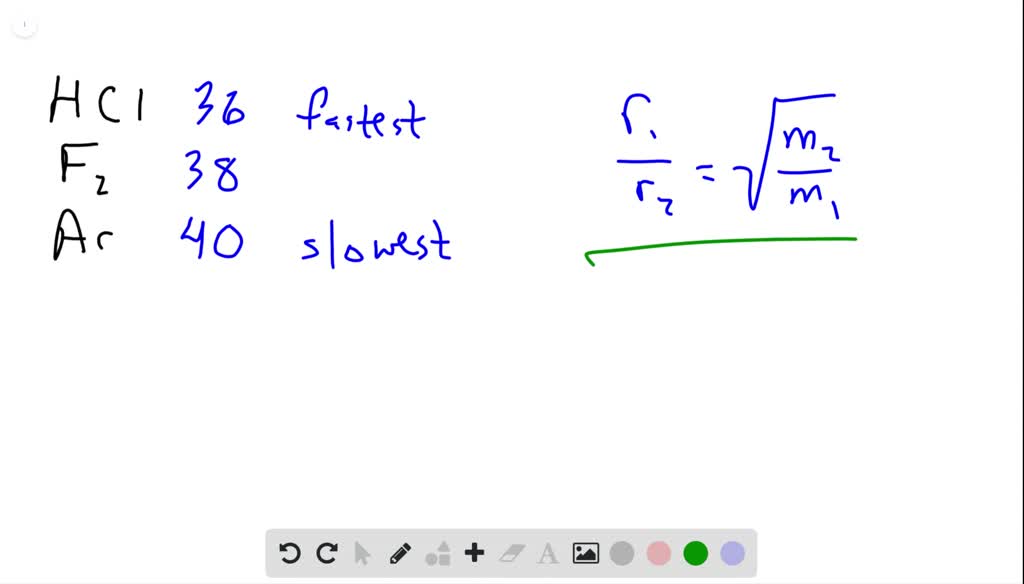
The rate of diffusion of 2 gases 'A' and 'B' are in the ratio 16:3. If the ratio of their masses present in the mixture is 2.3. Then A) The ratio of
Rate of diffusion of a saturated hydrocarbon is about 1/6 th of that of hydrogen under similar conditions of temperature and pressure. What is the molecular formula of that hydrocarbon?
Can the rate of effusion or diffusion be negative, in accordance with Graham's law? If so, how? - Quora
When r, p and M represent rate of diffusion, pressure and molecular mass, respectively, then the ratio of the rates of diffusion (rA/rB) - Sarthaks eConnect | Largest Online Education Community

Rates of diffusion of two gases A and B are in the ratio 2:1. If the molecular mass of gas A is 16g. Find the...
63.Rate of diffusion of gas X is twice that of gas Y if molecular mass of Y is 64 then the molecular mass of X will be

The rates of diffusion of gases A and B of molecular weight 36 and 64 are in the ratioa)9:16b)4:3c)3:4d)16:9Correct answer is option 'B'. Can you explain this answer? - EduRev JEE Question